Discovery of a potential therapeutic target to combat trypanosomes
Yaser Hashem's team at the Laboratoire Architecture et Réactivité de l'ARN at CNRS's has discovered a new potential therapeutic target – located in the ribosome – to combat trypanosomes parasites. Using cryo-electron microscopy1 , researchers at the Institut de Biologie Moléculaire et Cellulaire (CNRS/Université de Strasbourg) have analyzed the structure of these parasites in details and revealed one of their potential weak points, which has remained undetected until now. This discovery opens the path to the development of new safer therapies that are less toxic and more specific against trypanosomes, the parasites causing the Chagas disease and the African sleeping sickness. This study is published on October 26, 2017 in Structure.
- 1The 2017 Nobel Prize for Chemistry has just been awarded to Jacques Dubochet, Joachim Frank and Richard Henderson, for the development of this technique. Joachim Frank is the scientific mentor and a close collaborator of Yaser Hashem (they have co-authored 13 publications in high impact scientific journals): the discovery of this potential therapeutic target is a project that began at Columbia University (New York) when Yaser Hashem was in Joachim Frank's team.
Trypanosomes, more generally called kinetoplastids, are unicellular parasites responsible for numerous diseases of variable severity that can be lethal in the most severe cases. Trypanosoma brucei, Trypanosoma cruzi and Leishmania majorare probably the best known and cause the African sleeping sickness, Chagas disease and various Leishmaniasis, respectively.
Unlike bacteria, these organisms are eukaryotic cells that contain a nucleus, just like human cells. The similarities, though low, between animal cells and trypanosome cells complicate some therapeutic approaches. For example, an antibiotic targeting given molecular machinery in trypanosomes such as the ribosome could harm human cells at the same time. Until now, researchers thought that eukaryotic ribosomes (molecules involved in protein synthesis) had extremely similar structures from one species of eukaryotes to another, such as for instance the case of humans and trypanosomes, making them almost untouchable. Recent technological advances made possible the visualization of the structure of the ribosomes from trypanosomes at near-atomic resolutions, thus small structural differences to the human ribosomes can now be seen and become a potential therapeutic target.
Yaser Hashem's team has particularly looked at the architecture of the Trypanosoma cruzi ribosome. Using cryo-electron microscopy – involving sample cryogenization, it allows biological structures to be visualized in their native state – in combination with mass spectrometry – using the mass of each element to determine a precise protein composition – they have brought to light a protein specific to the ribosome of trypanosomes: KSRP (kinetoplastid-specific ribosomal protein). In addition to being specific to these parasites, KSPR is essential to their survival since inhibiting its activity leads to death of the parasites. The exact role of KSRP in the protein synthesis remains unsolved.
This discovery of KSRP gives us a glimpse into possible future medical research for the development of new therapies against trypanosomes parasites. Elucidating the structure of this new protein could lead to designing molecules that can interact with and inhibit its activity in a highly specific way, without interfering with host cells. So the possibility of targeting and inhibiting KSRP in parasites will represent a safer alternative, and especially a more efficient alternative, compared to current treatments that are extremely difficult and toxic.
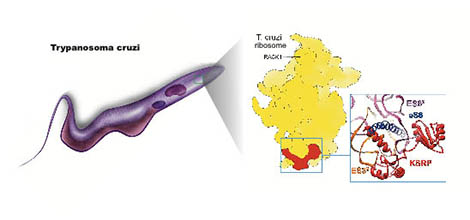
Ribosome of the parasite Trypanosoma cruzi. Illustration of the cytosolic ribosome in trypanosomes, purified from the T. cruzi parasite. Structure analysis by cryo-electron microscopy (middle) of the ribosome shows a new protein that is specific to this family of organisms. This protein has been named Kinetoplastid-specific ribosomal protein, or KSRP. In spite of its constant presence, this protein has passed unnoticed for years, even after the first publication of the high-resolution structure of this ribosome.
The cryo-EM structure of a novel 40S kinetoplastid-specific ribosomal protein. Jailson Brito Querido, Eder Mancera-Martínez, Quentin Vicens, Anthony Bochler, Johana Chicher, Angelita Simonetti, and Yaser Hashem. October 26, 2017, Structure. View web site


